
New Drug Designations - March 2024
Shots:
-
PharmaShots' designation report provides a concise overview of several drugs and their designations by the FDA, MHRA and EC. This month’s report includes designations allotted to 10 small molecules, 6 biologics, 5 cell & gene therapy, 1 recombinant fusion protein, 2 actineoplastics, 1 antidepressant, 1 drug conjugate, 1 analgesic and 6 devices
-
Trellis Bioscience's TRL1068 received FTD and QIDP designations from the US FDA for the treatment of Infectious diseases
-
PharmaShots has compiled a list of a total of 27 drugs and 6 devices awarded with designations by multiple regulatory bodies in Mar 2024


Avutometinib

-
The ODD has been granted to avutometinib alone or in addition with defactinib to treat recurrent low-grade serous ovarian cancer (LGSOC)
-
The drug is currently under P-III (RAMP 301) clinical evaluation with defactinib vs standard CT or hormonal therapy as a treatment of recurrent LGSOC
-
The P-II (RAMP 201) registration-directed study also investigates avutometinib + defactinib for recurrent LGSOC. The patient recruitment in the dose optimization, expansion phase & low-dose evaluation arms has been completed
-
The company anticipates the rolling NDA submission for accelerated approval in H1’24 and its launching in 2025
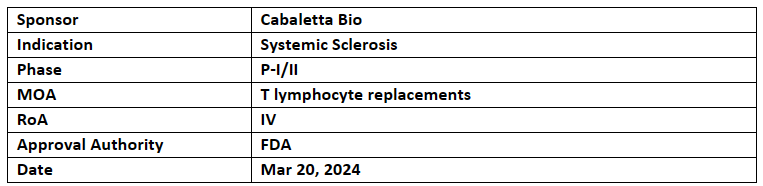
CABA-201
-
CABA-201 is being evaluated under the RESET (REstoring SElf-Tolerance) program consisting of 4 P-I/II studies for treating various autoimmune disorders incl. P-I/II (RESET-SSc) study
-
The P-I/II (RESET-SSc) program comprises a total of 9 arms with all arms starting at a dose of 1x10^6 cells/kg without the need of dose escalation
-
Cabaletta has gained the US FDA’s IND clearance of CABA-201 across various autoimmune conditions like systemic lupus erythematosus (SLE), myositis, systemic sclerosis (SSc), and generalized myasthenia gravis (gMG)
-
CABA-201 aims to temporarily deplete CD19+ B cells with a single infusion, leading to immune system reset for a lasting remission off therapy for autoimmune disease patients
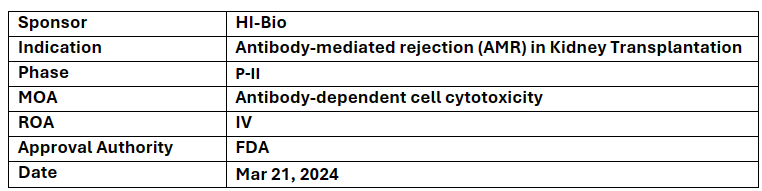
Felzartamab
-
HI-Bio’s Felzartamab has received ODD from the US FDA for AMR treatment in kidney transplant recipient and was already designated with the US FDA’s BTD for primary membranous nephropathy
-
In 2022, the company in-licensed felzartamab from MorphoSys and possess exclusive global rights excl. Greater China
-
Felzartamab is a monoclonal antibody that targets CD38 to improve clinical outcomes in a wide range of pathogenic antibody mediated disorders. It is being explored for other severe immune-mediated diseases, incl. IgA nephropathy (IgAN), lupus nephritis (LN) & primary membranous nephropathy (PMN)
Utidelone Injectable (UTD1)

-
The ODD was supported by the two P-II studies, one of them (n=17) assessing utidelone injectable + etoposide & bevacizumab resulted in 73% CNS ORR & 91% CNS CBR while the other one (n=46) evaluated utidelone injectable + bevacizumab & showed mPFS of 7.7mos. & 12mos. OS rate of 74.4% in HER2 -ve BCBM patients
-
The company further anticipates the expansion of utidelone’s clinical evaluations for treating lung cancer brain metastasis & glioma based on its potential to cross BBB due to insusceptibility to P-glycoprotein-mediated efflux & physicochemical features
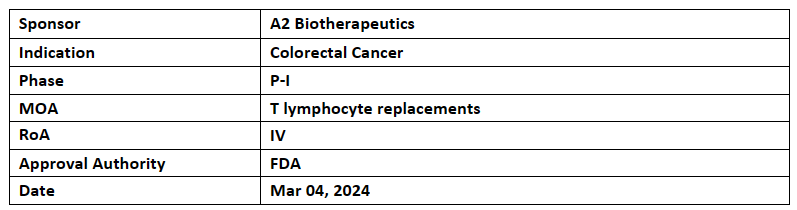
A2B530
-
The designation has been granted to A2B530 for treating germline heterozygous HLA-A*02(+) patients with colorectal cancer having carcinoembryonic antigen (CEA) expression and loss of HLA-A*02 expression
-
The company is evaluating A2B530 in the P-I/II (EVEREST-1) trial for its safety and efficacy to treat colorectal, pancreatic and non-small cell lung cancers
-
A2B530, based on Tmod platform, is an autologous logic-gated cell therapy which comprises of an activator targeting CEA and a blocker targeting HLA-A*02
UNI-494

-
The US FDA granted ODD to prevent delayed graft function (DGF) in kidney transplant patients
-
The company is currently assessing the drug under the P-I dose-ranging trial for its safety among healthy volunteers in UK and is expected to be concluded in H2’24
-
The preclinical data & P-I design were highlighted at the 29th International Conference on Advances in Critical Care Nephrology AKI and CRRT 2024
-
UNI-494, a cryoprotective agent, activates KATP channels in mitochondria restoring its function and prevent damage caused by ischemia/reperfusion injury. It is patent protected across the US & the EU
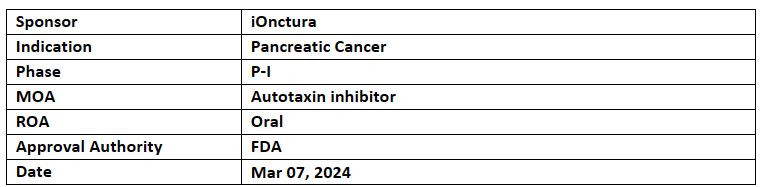
Cambritaxestat (IOA-289)
-
The company is assessing cambritaxestat under the P-I (AION-02) trial in combination with nab-paclitaxel and gemcitabine for the treatment of metastatic pancreatic cancer
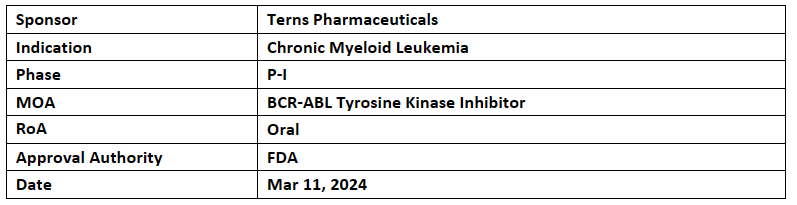
TERN-701
-
The company’s TERN-701 is under P-I clinical evaluation for treating CML
-
Interim results from initial dose escalation arms are anticipated in H2’24
-
TERN-701 is an in-house allosteric BCR-ABL tyrosine kinase inhibitor (TKI) which is presented being assessed for the treatment of CML
P-BCMA-ALLO1

-
Developed in partnership with Roche, P-BCMA-ALLO1 is being assessed under P-I, the results of which were highlighted at ASH 2023
-
The data demonstrated a well-tolerated and favorable safety profile in the ITT population without the need for bridging CT. Preliminary data showed the conversion of TSCM-rich CAR-T cells into cell-killing effector T cells that were sustained for at least 6wks. post treatment
-
The new data from recently enrolled patients were highlighted at AACR 2024 and the additional data is anticipated in H2’24
LYT-200

-
The company is assessing LYT-200 under P-Ib trial as monotx. And in combination with venetoclax and hypomethylating agents (HMA) for treating both AML & MDS. The initial data was revealed in 2023 and updated results will be highlighted at future conferences in 2024
-
LYT-200, a human IgG4 mAb, targets galectin-9, depicted direct cytotoxic, anti-leukemic effects & synergized with SoC CT & venetoclax in its preclinical studies
AP303

-
The company’s AP303 has shown renal survival improvement in an ADPKD mouse model.
-
Following the completion of FIH study among healthy subjects in Australia, it will now be evaluated under the P-II study for ADPKD
ImmCelz (CELZ-101)

-
CELZ-101 received ODD from the US FDA to prevent allograft rejection in patients undergoing pancreatic islet cell transplantation, hope for type 1 diabetes individuals
-
CELZ-101 is a cell-based immunotherapy that uses the patient's own Tregs to suppress rejection and potentially eliminate the need for lifelong immunosuppression after transplant
Tinengotinib

-
TransThera’s, tinengotinib has received ODD from the EMA for the treatment of biliary tract cancer (BTC) and was already designated with the US FDA’s ODD and FTD for CCA and in Jul 2023, it received the NMPA’s BTD
-
The company will now assess tinengotinib under the P-III (FIRST-308) trial for its safety & efficacy among cholangiocarcinoma (CCA) patients with FGFR-alteration, pretreated with CT- & r/r to FGFR inhibitor. The first patient has been dosed across the US in Dec 2023
-
Additionally, the P-/II clinical evaluation depicted the drug’s clinical benefit among the same patient population. The results were highlighted at ESMO 2023 & ASCO GI 2024

Nipocalimab

-
J&J’s nipocalimab has received the US FDA’s FTD to reduce the risk of fetal neonatal alloimmune thrombocytopenia (FNAIT) in pregnant women. It had also received the US FDA’s ODD for FNAIT in Dec 2023
-
The P-III (FREESIA) study assessing nipocalimab for treating FNAIT is underway
-
Nipocalimab is being studied hemolytic disease of the fetus and newborn (HDFN) under P-III studies
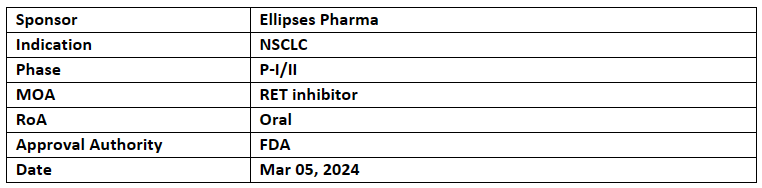
EP0031/A400
-
EP0031/A400 received FTD for the treatment of RET-fusion positive NSCLC, it also received ODD in Nov 2023
-
Data from interventional P-I/II showed tumour responses in NSCLC patients who were previously treated with first-generation SRIs.
-
Developed jointly by Ellipses Pharma (NCT05443126) globally and Sichuan-Kelun Biotech (NCT05265091) in China
LYT-200

-
The US FDA has granted FTD to LYT-200, human IgG4 mAb targeting galectin-9, in addition with tislelizumab to treat recurrent/metastatic HNSCC
-
The company’s LYT-200 is under the P-I/II trial as monotx. & combined with tislelizumab for advanced/metastatic solid tumors incl. HNSCC in which it demonstrated disease control, anti-tumor activity & a favorable safety profile across all cohorts
-
LYT-200 is also being investigated in the P-Ib study as monotx. & in combination with venetoclax & hypomethylating agents for treating hematological malignancies such as AML & high-risk myelodysplastic syndrome in which it showed a favorable safety & tolerability profile as well as potential clinical activity
Elritercept (KER-050)

-
The FTD has been granted to elritercept for the treatment of anemia in low or moderate MDS patients
-
KER-050 is an engineered ligand trap, combining the activin receptor type IIA ligand-binding domain with the human antibody's Fc domain
PT886

-
PT886 has also received the US FDA’s ODD for the treatment of pancreatic cancer in 2022
-
The P-I (TWINPEAK) study is currently assessing the safety, tolerability, PK/PD and preliminary efficacy of PT886 for treating locally advanced or metastatic G/GEJ and pancreatic cancers progressed post standard therapy or for which standard therapy was ineffective, intolerable or inappropriate
-
PT886 is a bispecific antibody, developed through PACbody and SPECpair platforms, targets claudin 18.2 and CD47 for treating G/GEJ and pancreatic adenocarcinomas

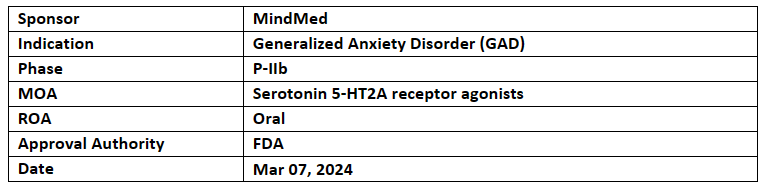
MM120
-
The BTD was based on the P-IIb (MMED008) study for GAD patients (n=198) who received MM120 (25, 50, 100 or 200µg) vs PBO. The full analysis set (FAS) for the trial comprised 194 individuals, all with at least one valid post-baseline HAM-A score
-
At wk.12, MM120 showed a 7.7-point improvement vs PBO (-21.9 vs -14.2), clinical response rate was 65% & clinical remission rate was 48% that sustained to wk.12. CGI-S scores shifted from 4.8 to 2.2 by wk.12, indicating substantial improvement.
-
The company anticipates End-of-P-II meeting with the US FDA in H1’24 and commencement of P-III study in the H2’24
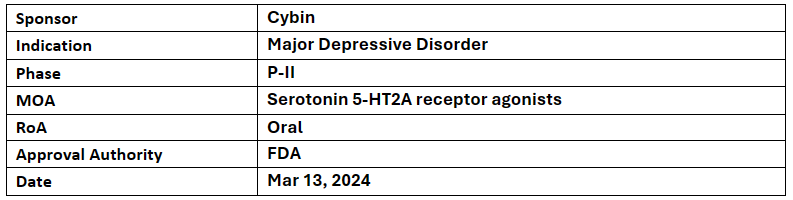
CYB003
-
The company’s CYB003 has received BTD as an adjunctive treatment of major depressive disorder supported by the data from CYB003’s (12mg or 16mg) P-II study in MDD
-
The results demonstrated a mean reduction of ~22 points in MADRS total score from baseline with both the doses, ~75% of patients responded after receiving 2 doses of 16mg and 60% (12mg) & 75% (16mg) of them were in remission from depression after 2 doses

PGN-EDO51

-
PGN-EDO51 received ODD and RPDD for treatment of DMD patients with exon 51 skipping mutation
-
PepGen is currently evaluating safety and efficacy of PGN-ED051 in ongoing CONNECT1 P-II trial. PepGen is planning to initiate recruitment for the P-II CONNECT2 study later in 2024
-
PGN-EDO51 is designed to skip exon 51 of the dystrophin transcript, an established therapeutic target for approximately 13% of DMD patients, thereby aiming to restore the open reading frame and enabling the production of a truncated, yet functional dystrophin protein.
LSTA1

-
Lisata Therapeutics received RPDD from the US FDA for LSTA1 for the treatment of Osteosarcoma
-
LSTA1, has demonstrated favourable safety, tolerability, and activity in clinical study for pancreatic cancer. It works by improving delivery of existing anti-cancer drugs to tumors making them more effective
-
LST1A has already received ODD for pancreatic cancer in the US and for glioblastoma in EU
TNX-2900

-
Tonix Pharmaceuticals TNX-2900 has received RPDD from the US FDA for the treatment of Prader-Willi Syndrome
-
TNX-2900 targets PWS, a genetic disorder causing uncontrollable hunger and obesity in children
-
TNX-2900 was previously granted ODD by the FDA in 2022 for the treatment of PWS and IND application was approved by the FDA in 2023
-
Tonix Pharma may be eligible for transferable priority vouched if TNX-2900 is approved for marketing

TRL1068

-
Trellis Bioscience's TRL1068 has Received FTD and QIDP designations from the US FDA for the treatment of Infectious diseases
-
TRL1068 targets prosthetic joint infections (PJIs) and other bacterial infections. It is designed to eliminate bacterial biofilms, making antibiotics more effective
-
P-I trial results shown TRL1068, is well tolerated with no serious side effects and eliminated bacterial biofilm in 7 days

P-Tau 217
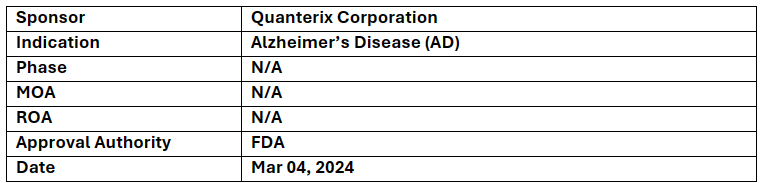
-
Quanterix’s p-Tau 217 blood test has been granted BDD by the US FDA as an aid in diagnostic evaluation of Alzheimer’s Disease (AD)
-
P-Tau 217 offer a simpler and less invasive approach compared to traditional methods like PET scans or spinal taps
-
The test is not meant to be a standalone diagnostic tool, intended to be used alongside other diagnostic tools to improve the evaluation of Alzheimer's risk, leading to earlier detection
Heart Disease Risk Staging System
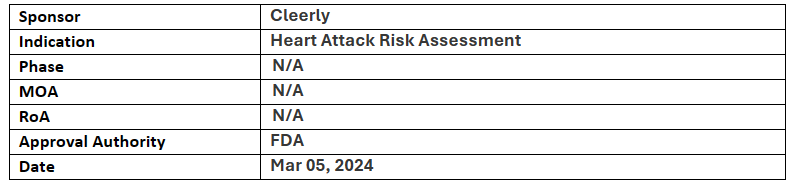
-
Cleerly’s CAD staging system receives BDD from US FDA; This software device analyses actionable features of coronary atherosclerosis, stenosis and ischemia and aids physicians for assessment of patients at risk of MACE
-
It is a non-invasive imaging-based investigational software device that analyses important and actionable features of coronary atherosclerosis, stenosis and ischemia
-
The Cleerly CAD Staging System will be evaluated in the TRANSFORM study which will recruit asymptomatic heart disease patients with diabetes, pre-diabetes or metabolic syndrome
-
The Cleerly CAD Staging System was also accepted into the FDA’s Total Product Life Cycle Advisory Program (TAP) Pilot
Neuroimmune modulation platform
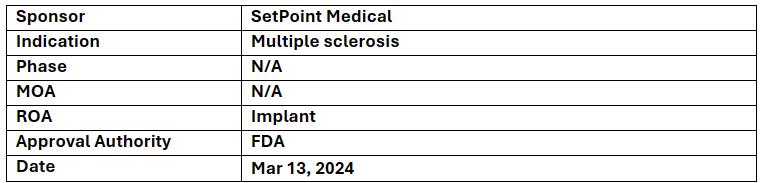
-
SetPoint Medical received BDD from the US FDA for their investigational platform technology for RRMS followed by prior (2020) BDD designation for the treatment of RA
-
First-of-its-kind approach, this therapy targets pathways intended to promote remyelination, which is the regeneration of the protective layer around nerve Fibers damaged by Multiple sclerosis
-
This device is placed near vague nerve via small outpatient procedure on the left side of the neck and programmed. It later stimulates the vague nerve once daily to activate the inflammatory reflex for a systemic immune-restorative effect
-
This platform technology is also undergoing a pivotal clinical trial (RESET-RA) to investigate its effectiveness as a treatment for adults with moderate-to-severe rheumatoid arthritis
Almee
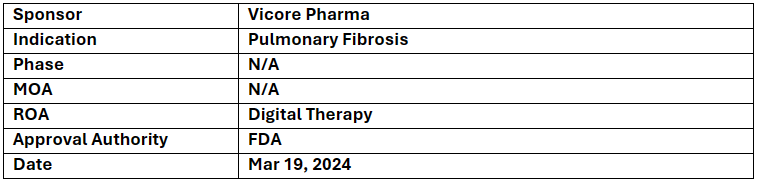
-
Vicore Pharma’s Almee a digital therapy has received BDD from the US FDA for patients with Pulmonary Fibrosis
-
The COMPANION study demonstrated a significant 2.7-point improvement in GAD-7 scores (measuring anxiety) and a positive impact on quality of life, with a 4.4-point increase in KBILD scores for patients using Almee
-
It is software built for patients to be used on smartphones or tablets designed to increase beneficial actions and improve quality of life. This tool is based on CBT (Cognitive Behavior therapy) principle used as adjunct to treatment of anxiety
Freesolve BTK RMS

-
Biotronik's Freesolve has received BDD from the US FDA for the treatment of CLTI
-
Freesolve BTK RMS leverages Biotronics's experience with Freesolve RMS (Existing Technology) for coronary arteries, which uses a biocompatible magnesium alloy that degrades over time
DynamX BTK System

-
Elixir Medical's DynamX BTK System received BDD from US FDA for the treatment of CLTI
-
The DynamX Bioadaptor has been shown to provide high acute lumen gain in coronary vessels and maintain such gain over time, which is a common challenge in existing below-the-knee (BTK) therapies
-
DynamX Bioadaptor is the first implant technology designed to support and then "uncage" a diseased vessel, promoting blood flow and preventing amputation

Sebetralstat

-
KalVista Pharmaceuticals has received Promising Innovative Medicine (PIM) designation for sebetralstat from UK MHRA for the treatment of hereditary angioedema (HAE)
-
Sebetralstat has already received FTD and ODD from the U.S. FDA, as well as ODD and an approved Pediatric Investigational Plan from the European Medicines Agency (EMA)
-
Recent P-III data showcased clinically and statistically significant results, along with a good safety and tolerability profile

BJT-778
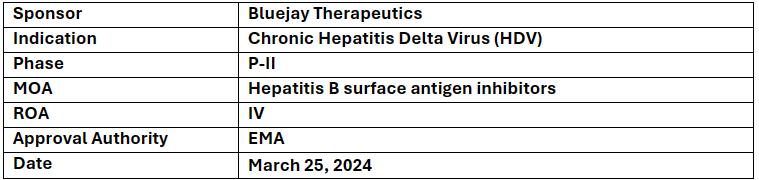
-
Bluejay's BJT-778 has received European Priority Medicine (PRIME) Designation from EMA for the treatment of Chronic Hepatitis Delta Virus
-
Bluejay's application was supported by positive non-clinical and interim results from their ongoing P-I/II study
-
BJT-778 works as a high-potency antibody that neutralizes and helps clear HDV virus from the body

PCRX-201
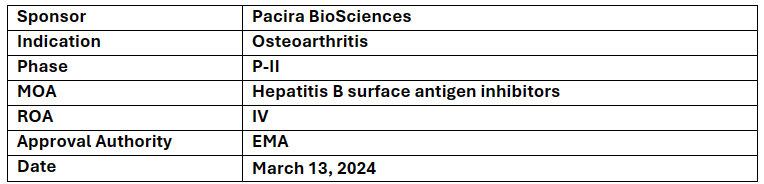
-
Pacira BioSciences’s PCRX-201 has received RMAT designation from the US FDA for the treatment of Osteoarthritis and was also granted Advanced Therapy Medicinal Products (ATMP) designation by the European Medicines Agency in May 2023
-
PCRX-201 in P-I study showed (n=72) to be well tolerated with positive efficacy observed for at least 52 wks. 50% improved in the WOMAC pain and stiffness score and a meaningful improvement in KOOS functional assessment in PCRX-201+ steroid arm
-
The 52-week data have been accepted for presentation at OARSI 2024 and the company expects to present 104-week efficacy and safety data later in 2024
Related Post: New Drug Designations - February 2024
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














